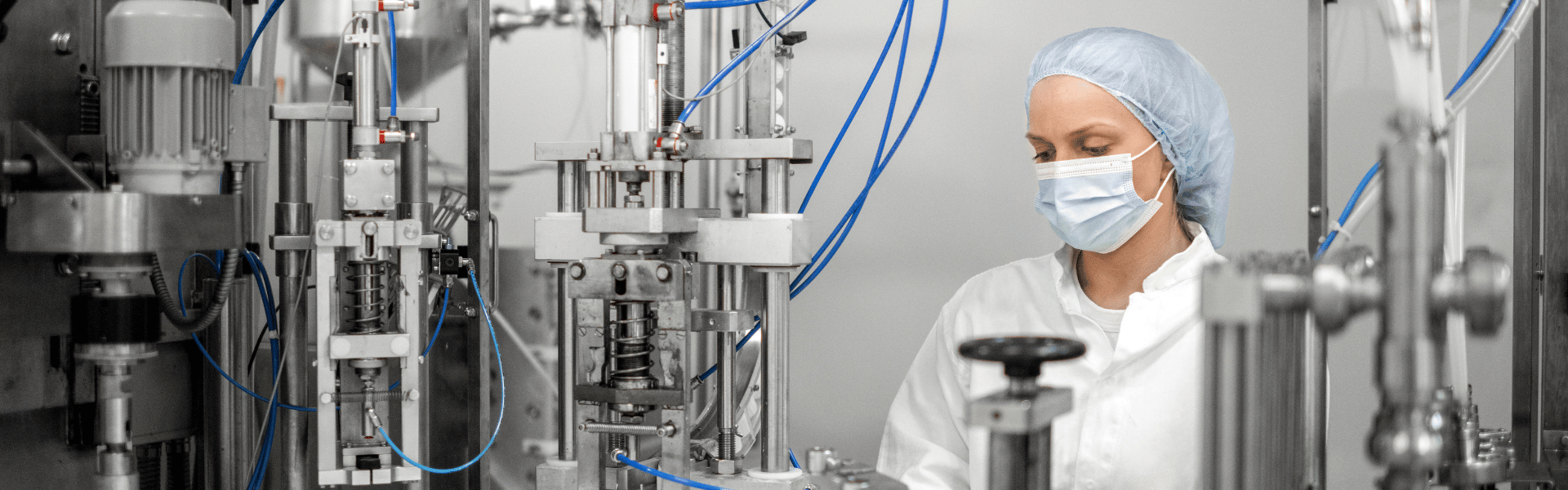
New Equipment to Triple Production Capacity of GMP-Compliant Raw Materials for Pharmaceutical Manufacturing.
FDA ensures the quality of drug products by carefully monitoring drug manufacturers' compliance with its Current Good Manufacturing Practice (cGMP) regulations. The cGMP regulations for drugs contain minimum requirements for the methods, facilities, and controls used in manufacturing, processing, and packing of a drug product. The regulations make sure that a product is safe for use, and that it has the ingredients and strength it claims to have.[1]
Need to increase the production
When scaling up from clinical trials in Phase I to large-scale commercial manufacturing, the number of patients and doses to be produced might go up by several magnitudes. As a consequence the supply of high-quality GMP grade raw materials is getting even more critical. Providing raw materials at a consistent high quality at highly increased amounts could form a challenge for your raw material supplier.
Solution
The newly installed equipment will cater to the increasing demand for raw materials in pharmaceutical manufacturing, such as stabilizers and buffers. This demand is driven by the growing need for biopharmaceuticals, which is expected to increase by approximately 10% annually due to the rising demand for antibody and gene therapeutics.
The raw materials pharmaceutical manufacturing sector benefits greatly from FUJIFILM Wako Pure Chemical Corporation's extensive range of products and services. Our "CertiPro" series deserves special mention, as it offers an assortment of raw materials that adhere to GMP regulations. These materials have important uses in key areas such as stabilizers and buffers, playing a vital role in the pharmaceutical manufacturing process. Furthermore, the Company offers customized "Bioprocessing Solutions" that cater to the specific concentrations and volumes needed for diverse biopharmaceutical manufacturing processes.[2]
Key features
- Multiple 20,000-L bioreactors : Multiple 2,000 L bioreactors Options for multiplexing (2 x 2000L)
- Flexible strategy mitigates risks due to uncertainties in commercial demand
- Ideal for lower volume products
- Continuous Single or multiple 500-L bioreactors or larger Efficiency of scale
- Ideal for high-volume products
- Improved production efficiencies Flexibility and potential to deliver high-volume
Uses
The newly installed equipment is capable of producing:
- Raw materials for pharmaceutical manufacturing (GMP compliant)
- Customized products of raw materials for pharmaceutical manufacturing
- Raw materials for pharmaceutical manufacturing (GMP compliant /ISO9001 compliant)
- Life science reagents (PCR, DNA extraction kits, etc.)
- Chemical reagents (powder and liquid reagents)
- Medium-related reagents (powdered and liquid media, process solutions)
- Clinical diagnostic reagent.[2]
References
- Food, U., et al., Current good manufacturing practice (CGMP) regulations. 2020.
- FUJIFILM Diosynth Biotechnologies CDMO: Multifunctional Single-Use Purification System for connected and integrated Continuous Processing. GMP pharma congress, 2022.